Abstract
Introduction: Philadelphia-chromosome positive (Ph+) acute lymphoblastic leukemia (ALL) is a high-risk subtype of ALL that affects 5% of pediatric and 25% of adult patients. Current therapy includes multi-agent chemotherapy or immunotherapy with an ABL-class tyrosine kinase inhibitor (TKI). Patients with relapsed/refractory disease do poorly. We and others have previously reported preclinical efficacy of venetoclax/TKI combinations in CML [PMID 27605552], a disease that shares an oncogenic driver with Ph+ ALL. We hypothesized that the addition of venetoclax to TKIs would also be effective in preclinical models of Ph+ ALL.
Methods: The human cell line SUP-B15 which harbors the minor BCR-ABL fusion was used for initial experiments and mechanistic studies. A syngeneic mouse model of Ph+ ALL involving murine cells transduced to express p185 BCR-ABL and the dominant negative mutant of IKZF1 with exons 3-6 deleted (IK6) was provided by Dr. Charles Mullighan (St. Jude). These cells generate an aggressive leukemia in C57Bl/6 mice with latency of 3 weeks. CD45.2 p185-IK6 cells were injected into CD45.1 C57Bl/6 mice and engraftment as well as mouse survival in the presence or absence of venetoclax and dasatinib was assessed. Four human Ph+ ALL specimens (2 adult, 2 pediatric) were also employed for in vitro and in vivo (xenograft NSG-S model) assessment of anti-leukemia effects of venetoclax, dasatinib, or the combination. Cytotoxicity was measured through flow cytometry using an antibody to annexin V and the cell-impermeable DNA intercalator DAPI. Double-negative cells (annexin V-negative DAPI-negative) were quantified as alive and reduction in live population in drug-treated cells was quantified relative to vehicle. For in vivo experiments percent of leukemic bone marrow engraftment and mouse survival were used as endpoints to compare efficacy of drug combinations to vehicle-treated mice. The Agilent SeaHorse XFe96 Analyzer was used to evaluate energy production in leukemia cells treated with sub-lethal doses of venetoclax, dasatinib, or the combination.
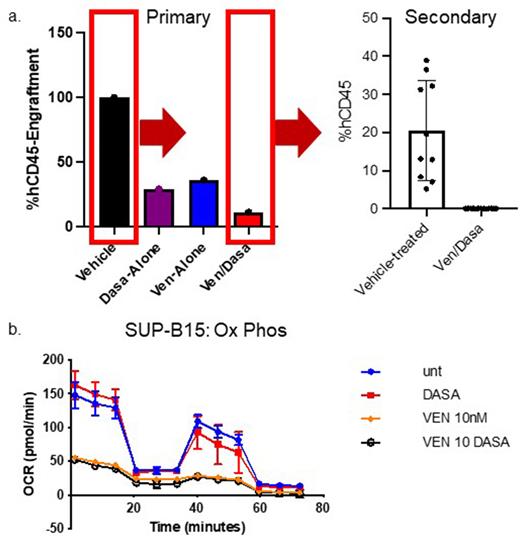
Results: The combination of venetoclax and dasatinib resulted in a significant reduction in viability of the SUP-B15 cell line, p185-IK6 mouse leukemia cells, and all 4 samples tested via xenograft as compared to vehicle or either drug. In vivo treatment with venetoclax/dasatinib significantly prolonged survival in the syngeneic mouse model of Ph+ ALL and reduced engraftment in our xenograft model (Figure 1a) compared to vehicle or either drug alone. Secondary transplant of leukemia cells from combination-treated mice failed to engraft in recipient NSG-S mice (Figure 1a). No effect of venetoclax/dasatinib was seen on hematopoiesis of normal murine HSCs. Inhibition of cellular capability for oxidative phosphorylation was seen in venetoclax- and venetoclax/dasatinib-treated SUP-B15 cells at full viability (Figure 1b), similar to what has been previously described in acute myeloid leukemia (AML) patient samples.
Discussion: Venetoclax + dasatinib demonstrated more effective inhibition of Ph+ ALL than either drug alone in preclinical models of disease. Transplantation of combination-treated cells into secondary recipient mice did not lead to appreciable engraftment, suggestive of a depletion of functional leukemia-initiating cells upon exposure to venetoclax + dasatinib. As has been seen with AML, venetoclax targets energy metabolism in Ph+ ALL. Comprehensive mechanistic investigations of the venetoclax/TKI combination in Ph+ ALL are ongoing; however, these data provide a biologic rationale for integration of venetoclax into therapeutic regimens for this high-risk leukemia.
Disclosures
No relevant conflicts of interest to declare.
Author notes
∗Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal